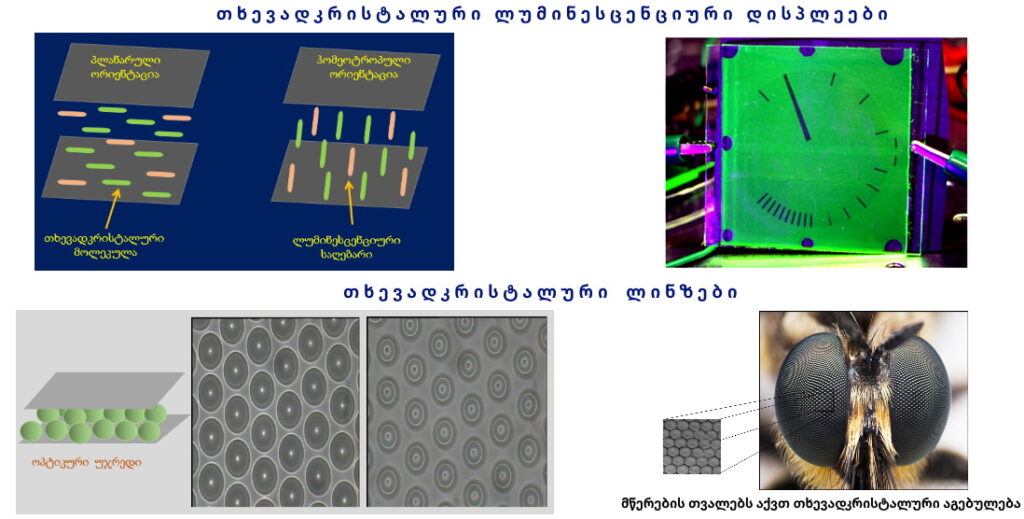
Laboratory of thermo- and photochromic structures






Thermochromism is the property of substances to change color due to a temperature change. Thermochromicmaterials change color reversibly with temperature changes. They can be made as semi-conductor compounds, from liquid crystals or metal compounds.
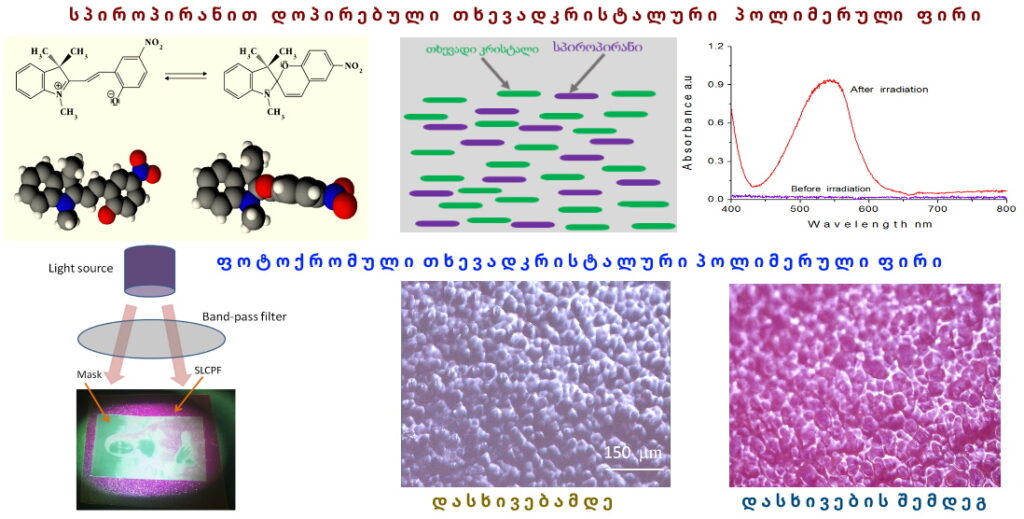
Photochromism is the reversible transformation of a chemical species between two forms by the absorption of electromagnetic radiation, where the two forms have different absorption spectra. Trivially, this can be described as a reversible change of color upon exposure to light. Photochromism is the light-driven reversible transformation between two isomers possessing different absorption spectrums and geometrical structures.
Azobenzene, spiropyran, phenoxyquinone, and bisthienylethene derivatives are representative of the most common types of photochromic compounds explored to date. One of the most unique examples of molecular switches is spiropyran (SP), whose closed-ring form, a hydrophobic isomer transforms into a highly polar, open-ring merocyanine (MC) forms, upon exposure to UV light, whereas the reverse reaction can be induced by visible light, or by heat.
Liquid Crystals: Thermo- and Photochromic Properties and Their Modern Applications

Liquid crystals represent an intermediate phase state between solids and liquids. Like solids, they exhibit anisotropy with respect to optical, electromagnetic, and acoustic fields, while, like liquids, they possess fluidity.

Liquid crystals are divided into nematic, smectic, and cholesteric phases, which differ from each other based on molecular arrangement.
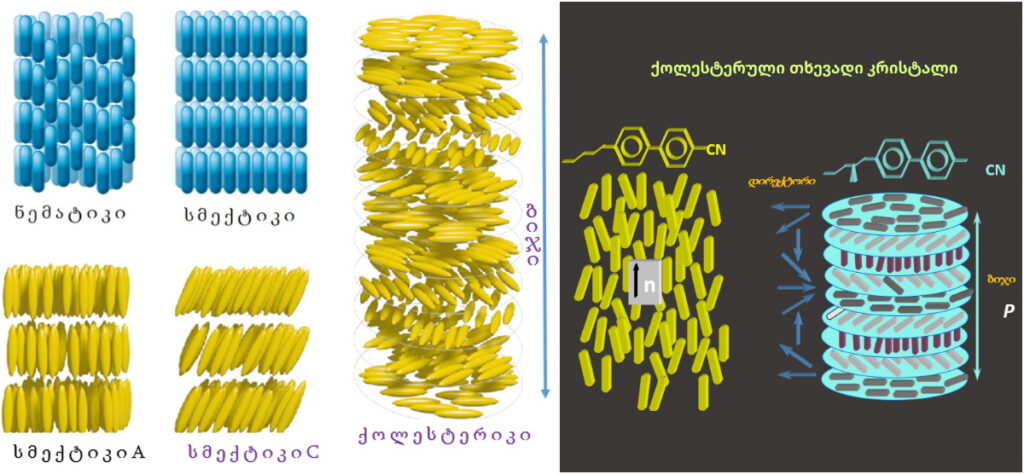
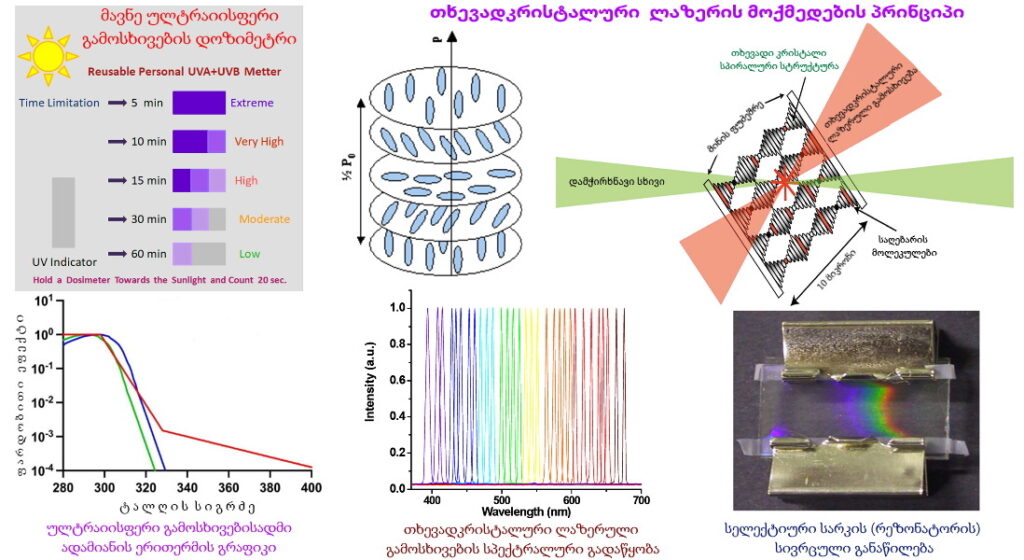
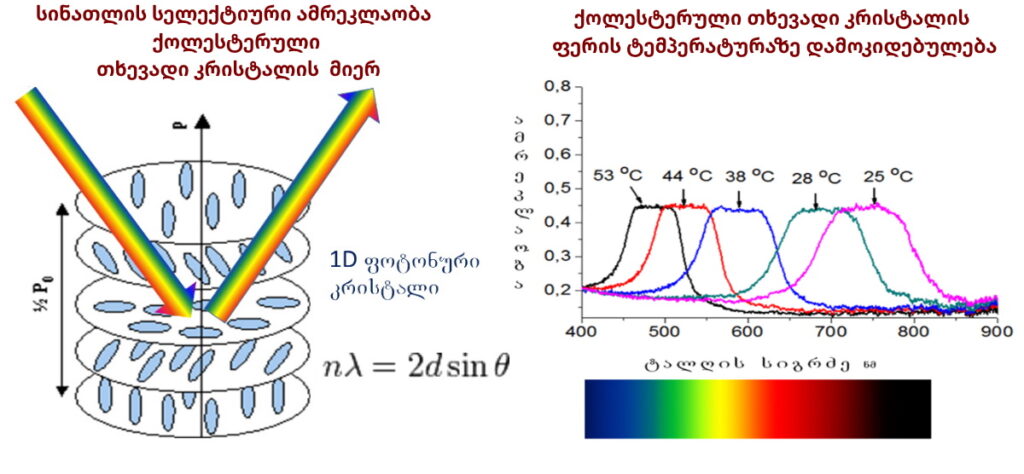
Liquid Crystalline Blue Phases
Blue phases are composed of three-dimensional cylindrical structures made up of double-twisted spirals. There are three main blue phases: BPI, BPII, and BPIII (Fog), which differ in symmetry. Blue phases exhibit narrow selective reflectivity, controlled by temperature or electric fields.
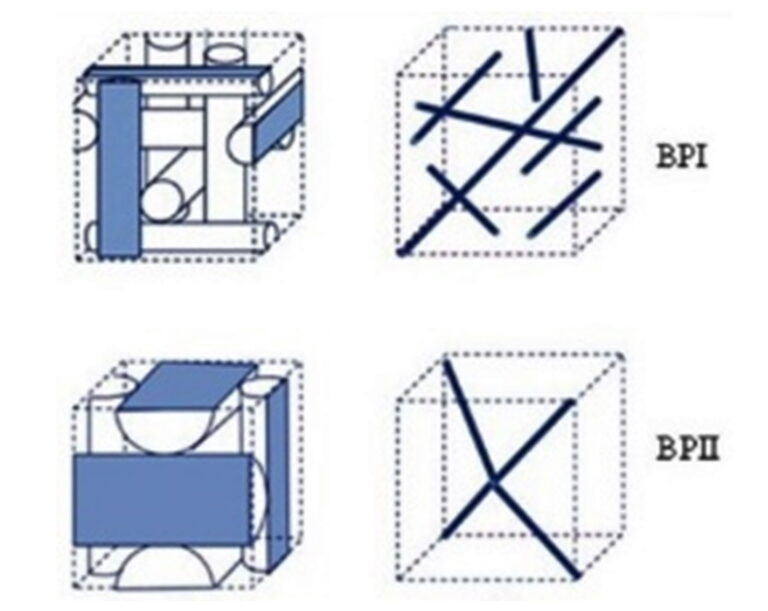
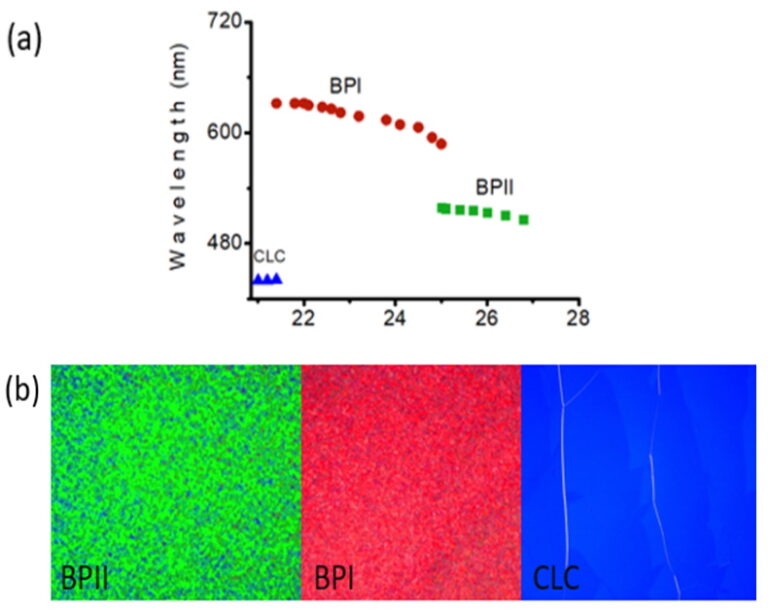
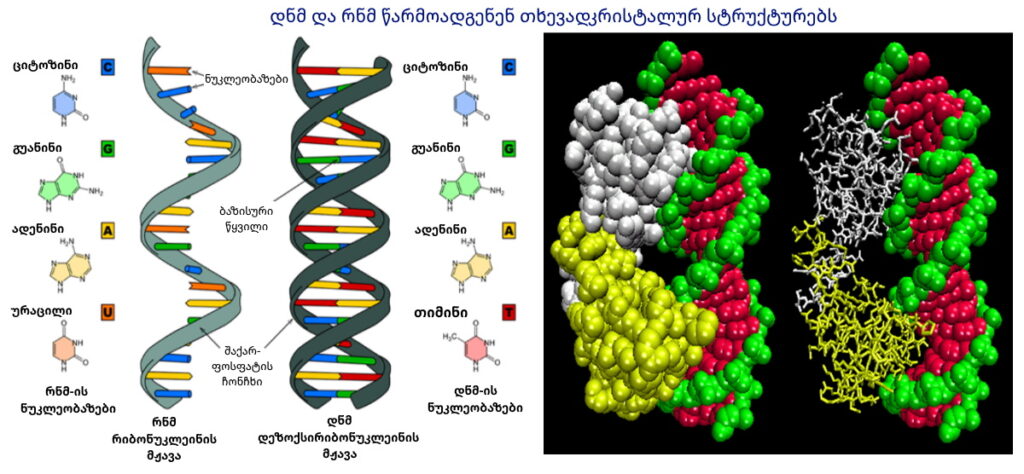
Liquid crystal phases
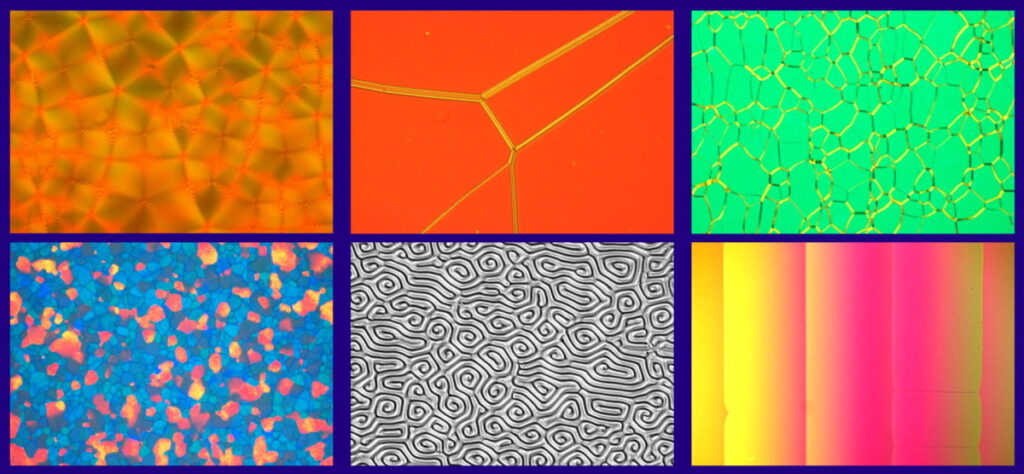
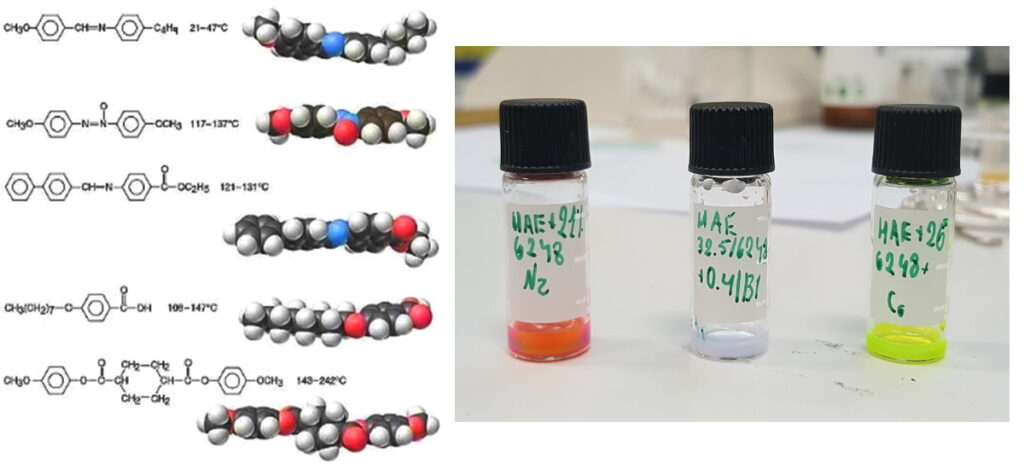
Liquid Crystalline Molecules and Fabricated Samples
Liquid Crystalline Biological Structures
Liquid crystalline structures are found in liposomes, micelles, and even viruses.

Liquid Crystalline Structures in the Living World

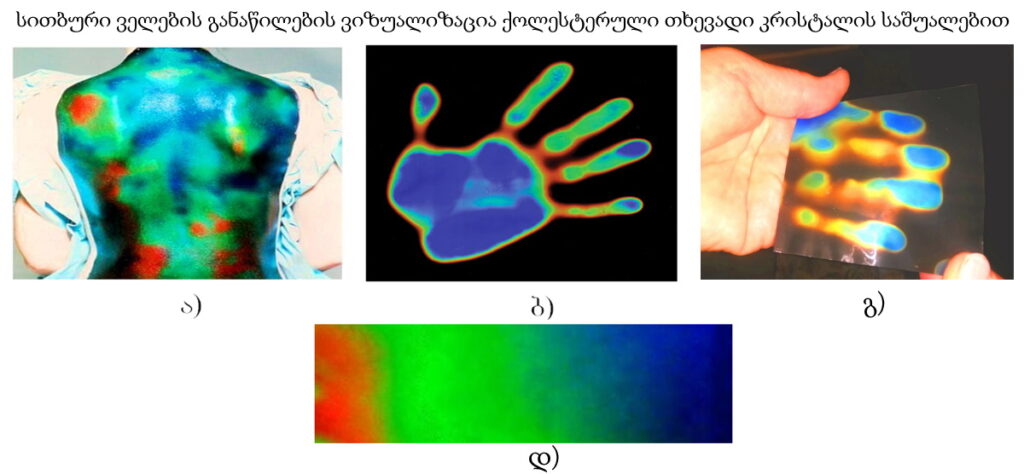
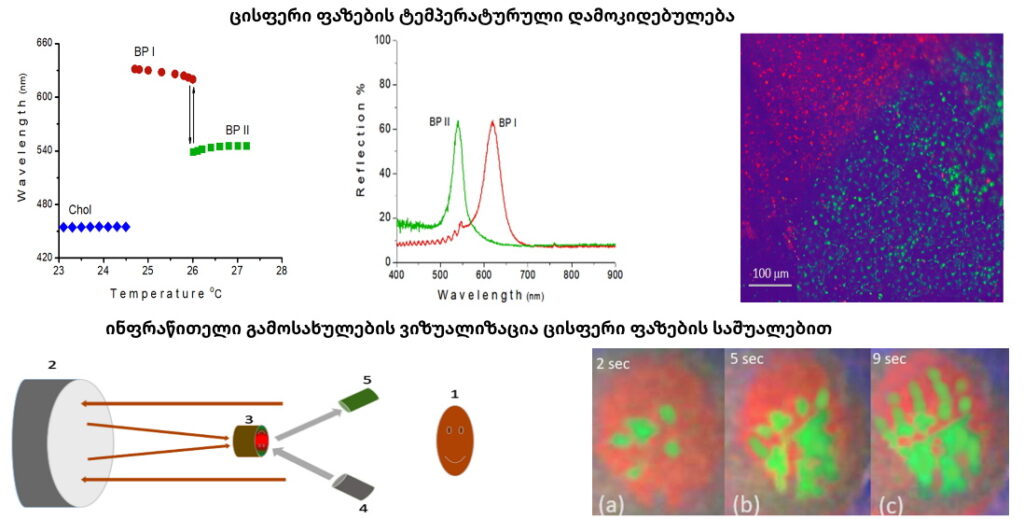
Environment-Adaptive, Temperature-Controlled Cholesteric Liquid Crystal Mirrors for Energy-Saving and Energy-Generating Smart Windows
Approximately 30–40% of global energy is consumed in buildings for heating, cooling, lighting, and ventilation. Energy consumption in buildings is particularly dominant in hot and humid regions, accounting for one-third to half of all generated electrical energy. Smart windows are systems capable of sensing and responding to environmental stimuli such as light, heat, or electricity. They control the light passing through the glass, allowing reversible regulation of light and heat inside buildings. This makes them a foundation for next-generation, multifunctional devices.
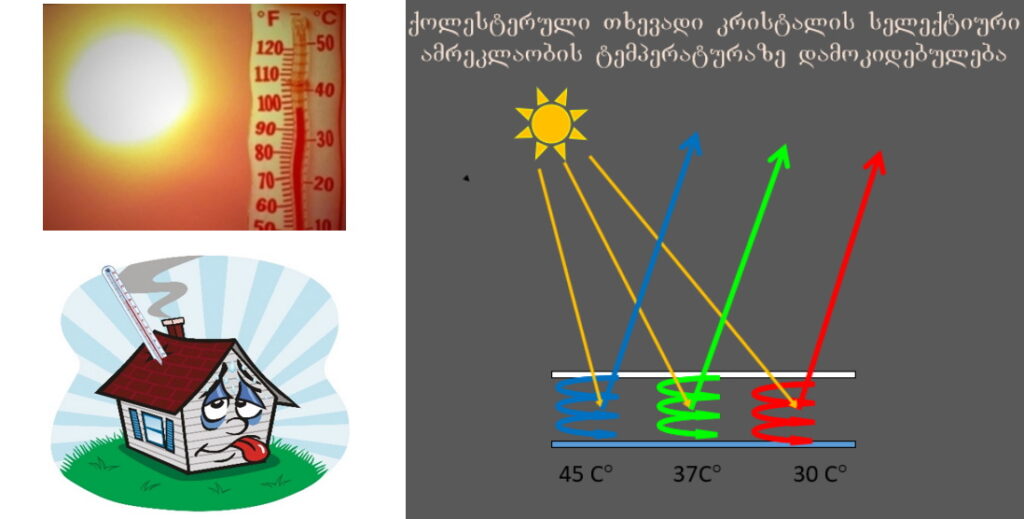
Controlling Window Transparency with Temperature-Self-Regulating Smart Windows

Reflective Liquid Crystal Displays
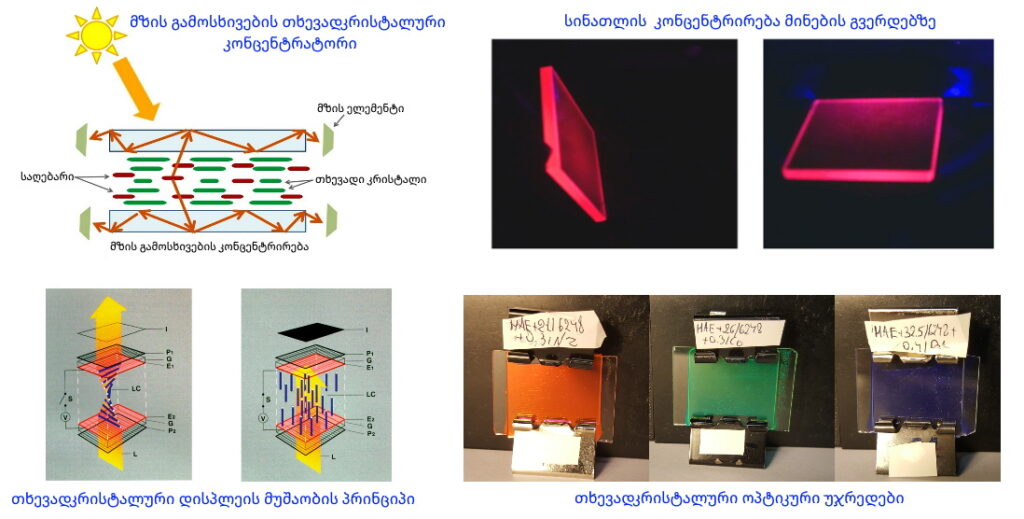
Currently, the vast majority of liquid crystal displays utilize only 4–6% of transmitted or reflected light, which is highly inefficient from an energy perspective (see Fig. A). To address this issue, leading laboratories worldwide are actively working on developing displays that use external light sources (e.g., sunlight, lamps) for information visualization.
A new type of reflective liquid crystal display has been proposed, distinguished from existing analogs by high contrast and resolution, operation at low voltages, simple manufacturing technology, and low cost (see Fig. B, Fig. C, Fig. D). Based on this technology, information display devices of any size and shape can be produced.